Digression 3: CORROSION - Rust Never Sleeps

Autumn in Pennsylvania at a “Volkswagen Graveyard.” As soon as the snow flies, the Pennsylvania Department of Transportation will begin spreading its annual 1 million tons of rock salt on the roads to melt the snow and ice, thereby hastening the state’s 8 million vehicles on their way to an early grave from rust.
DEFEATING RUST is an endless drudgery of oiling, greasing, brushing, scraping, cleansing, swabbing, painting, repainting, re-repainting, and finally discarding. All that hassle and expense is a strong incentive to devote a great deal of science and engineering to improve the situation. Thus maintenance issues are the force behind the anti-corrosion part of technological progress. We want to make rust just go away, and it just won’t. So we negotiate.
Later editions of John Muir’s opus on Volkswagen repair conclude with a section titled “How To Keep Your VW Alive Forever.” The book emphasizes that the most crucial requirement is to “keep rust at bay.”1 Storing the car is no solution because it will suffer from what dealers call “lot rot.” Since rust thrives in moist conditions, humid climates are deadly. The most corrosive regions are near the salty moisture in ocean air or on icy roads strewn with salt to make driving safer. In America’s humid southeast and freezing northern states, few old VWs can be found still running. They’ve all rusted out.
John Jerome rants in his book Truck,
Road salt makes a mushy, corrosive paste that is flung universally about the under- and over-sides of every vehicle. It fouls all the metal parts, pits windshields, scours paint, and reduces the useful life of north-country motor vehicles by several years. It also kills roadside trees, pollutes streams and wells, and destroys gardens.2He wrote that in 1977. It’s still true. There must be a way to ensure safe driving in snow that doesn’t destroy every vehicle on the road, but so far all the less harmful substitutes for rock salt, such as sugar beet juice, cost four to ten times more than rock salt’s $60 a ton.
People have always agonized about their cars and trucks rusting out. It used to be said of Model T’s that “on a quiet night, you can hear a Ford rust.” That’s plausible, according to Jonathan Waldman’s book Rust. He writes:
Because corrosion is exothermic, the skin of a corroding Ford becomes hotter than the metal underlying it, and this thermal gradient generates local stress called electrostriction. Technically, with the right tools, you really could hear it.3“Exothermic” means it emits heat. Rusting is slow fire—an oxidation process that converts iron or steel exposed to air and moisture into iron oxide. One definition of corrosion describes it as “extractive metallurgy in reverse…. Iron is made from hematite by heating it with carbon. Iron corrodes and reverts to rust, thus completing its life cycle. Hematite and rust have the same composition.”4
From rust to rust, with civilization along the way.
Metallurgy and civilization ascended together. Because metals are hard, tough, dense, shiny, recyclable, and—most importantly—shapeable, they proved perfect at first for making prized adornments, tools, and weapons, and later for civilization’s extensive structures, machines, pipes, and fasteners. Nearly everything made by humans has metal in it. So many billions of tons of metal are deployed worldwide that its corrosion is a planet-scale expense. According to a 2016 report by the National Association of Corrosion Engineers, “The global cost of corrosion is estimated to be US$2.5 trillion a year, which is equivalent to 3.4 percent of the global Gross Domestic Product.”
The report earnestly continues, “By using available corrosion control practices, it is estimated that savings of between 15 and 35% of the cost of corrosion could be realized, i.e., between US$375 and $875 billion annually on a global basis.”5 That’s the happy news. The hard news is what the engineers are admitting: even if every one of their best practices is deployed worldwide, corrosion will still cost the world between 2.2 and 2.9 percent of global GDP—$1.6 to $2.1 trillion.

NINE TYPES OF CORROSION
Like cancer, corrosion comes in a variety of forms, each requiring a different mode of detection and treatment. 1) Atmospheric corrosion - caused by moisture in the air. 2) Erosion corrosion - caused by mechanical abrasion from corrosive fluids, common in piping. 3) Selective corrosion - occurs in alloys where one of the metals, such as the zinc in this brass pipe, is dealloyed selectively. 4) Uniform corrosion - where the lack of a protective coating causes the whole surface to corrode uniformly. 5) Pitting corrosion - caused by localized damage to a protective layer or by structural defects in the metal. 6) Galvanic corrosion(also called bimetallic corrosion) - caused by direct contact between different metals (here, stainless steel bolts on zinc-galvanized steel). 7) Stress corrosion - caused by a combination of mechanical stress and a highly corrosive environment such as chloride or ammonia. 8) Inter-granular corrosion - occurs at grain boundaries in the metal, caused by differential heating such as in defective welding (“grain” is the tiny crystals in metal formed during cooling). 9) Corrosion fatigue - where metal fails due to constant mechanical stress and corrosion.

Civilization is in the same precarious situation as Robin Knox-Johnston on his sailboat SUHAILI in the stormy Southern Ocean. His engine rusted solid from just a bit of neglect. Constant vigilance, labor, and ingenuity are required to keep all our engines from rusting solid, our bridges from collapsing, and our pipelines from failing catastrophically. Dealing with corrosion is such a massive and crucial undertaking that the search for new ways to do it better never stops. Most of the techniques discovered so far involve trying to make a protective coating so perfect that no combination of moisture and air—or any other corrosive material—can get at the metal.
Paint on steel is the commonest. Why steel? It’s cheap and exceptionally sturdy. Its primary ingredient, iron, is abundant in the Earth’s crust. Iron is relatively inexpensive to extract from ore, and relatively inexpensive to smelt with enough carbon to make steel. Just since 1950, civilization has produced 36.8 billion tons of steel, according to the World Steel Association.6 The US alone makes a quarter-ton of steel per person per year—three-quarters of it recycled from scrap. The resources analyst Vaclav Smil rightly titles his book on steelmaking Still the Iron Age.7—we’ve been smelting iron for 3,000 years.

The “Rust Wedge” at the Exploratorium in San Francisco demonstrates how rusting iron expands so powerfully it can crack concrete. This photo was taken in February, 2012—just 3 years and 4 months after the museum put the concrete block and its iron plates out in the rain. (The Rust Wedge was dismantled in 2015 when the Exploratorium moved and was not recreated at the new site.)
Undermining the virtues of all that steel is one terrible flaw: iron oxide is a bigger molecule than iron. When rust forms, it puffs up and flakes off, exposing ever more of the steel to rust and flake off. When steel rebar rusts inside reinforced concrete, the puffing up crumbles the concrete. Copper and aluminum corrode more benignly—their oxides protect the metal by bonding tightly to it—but they are so much weaker and more expensive than steel that the endless hassle of painting steel is the better choice most of the time.

Thin layers of paint skillfully applied are all that stand between the chaos of rust and the fragile order of a still-floating steel ship. The average large cargo ship requires 28 tons of paint—repainted every 5-10 years. If the ship carries 20,000 containers, they require 550 tons of paint.
Bernard Moitessier’s obsession with getting the best possible layering of specialized paints to protect his steel sailboat JOSHUA exemplifies the pains people take to seal off steel from an oxidizing world. It is an ultra-specialized field. In the course of research for his book Rust, author Jonathan Waldman found the most thorough appreciation of the arcana of paint in an organization that estimates its annual corrosion costs at $500 billion—the US military. The lore he discovered at the Pentagon inspired him to write litanies worth reciting aloud. “The Corrosion Protection Office,” he reported,
has funded the development of coatings for aircraft, decks, fire systems, jet fuel tanks, water tanks, air-conditioning coils, pump impellers, vehicle underbodies, bilges, magnesium parts, and cold environments. They’re single coats or multiple coats, primers or topcoats, designed to cure quickly or at high temperatures or low temperatures, for spraying or rolling or powder coating or depositing by laser. Some are magnesium rich, or zinc rich, or vinyl based, or epoxy based, or nickel titanium based, or specifically chrome free. Some are fluorescent, stealth, sticky, thick, long-lasting, flexible, fire-resistant, chip-resistant, thermally insulating, or nonskid. The office has put more than $3 million toward paints that are self-priming, self-inspecting, self-cleaning, or self-healing.8 Engineers at the Naval Research Lab composed an ode to the paint on their nuclear submarines:
The purpose of the hull is to protect the paint.The purpose of the reactor is to drive the paint around.The purpose of the SUBSAFE program is to ensure the paint comes to the surface and will not be lost.The purpose of the cathodic protection system is to back up the paint.The purpose of the weapons is to defend the paint.The purpose of the Special Hull Treatment is to protect the paint.The purpose of the Vertical Launch System is to destroy those who would do the paint harm.9High expertise is required to find the right paint for a job and then apply it in exactly the right way. When it goes wrong, you need further expertise to analyze the problem and correct it. The best guide is a publication from the National Association of Corrosion Engineers titled Fritz’s Atlas of Coating Defects. Waldman’s Rust book chants what is included:
A coating may be cheesy, checked, rippled, wrinkled, peppery, seedy, saponified, crocodiled (or alligatored), cratered, crazed, cobwebbed, crow’s-footed, or cracked, like mud or stars. It may look, technically, like an orange peel. It may appear flocculated, or have fish eyes, or be flaking. It may be blistering, bubbling, cissing, disbonded, delaminated, pinholed, peeling, or just plain undercured.10
No wonder everyone longs for a single comprehensive protective coating that can last indefinitely with no need for maintenance at all, please. A coating that comes close to that ideal is hot-dip galvanized steel. The magic ingredient is zinc. When you dip ordinary steel in an ultrahot bath of molten zinc (840 degrees Fahrenheit), you get an inexpensive product with impressive properties. The inner surface of the thin zinc layer bonds tightly with the steel as an alloy, and the exterior reacts with air to form non-corroding zinc carbonate.

A hot-dip galvanized steel wall remains intact while the ordinary steel letterbox mounted on it rusts away. The crystalline shapes on the galvanized surface, called “spangles,” are formed when the molten zinc cools.
Painted steel is a little cheaper than galvanized at first, but over time it costs three times more because it has to be periodically repainted, and galvanized doesn’t. Of the roughly two billion tons of steel produced annually in the world, as much as a fifth is galvanized steel, used primarily in construction.
For the billion people who live in shantytowns throughout the developing world, sheets of galvanized corrugated steel are their most treasured shanty-building material. In Machete Season, a book about the 1994 genocide in Rwanda, author Jean Hatzfeld has a section titled “Some Thoughts on Corrugated Metal.” He notes, “Of all the elements of the house…, the corrugated metal sheet is the only one the villager cannot make with his own hands — hence its commercial value.”11 The sheets don’t just have value; they are value. With two sheets you can buy a goat, with twenty an Ankole cow. Sheeting that is worn out from use as roofing and walls, writes Hatzfeld, “serves secondhand to build kitchen shelters, toilets, animal pens, and silos in the courtyard. It also enters into the making of doors, shutters, cabaret terraces, chests, and coffins for the poor.”12

Entirely clad in galvanized corrugated steel sheets, Migingo Island is in Africa’s Lake Victoria. Over 130 Kenyan and Ugandan fishermen occupy the half-acre island, served by four pubs, several brothels, a hotel, and a pharmacy. This photo was taken in 2016.
Galvanized steel is really good for a while, but only a while. Its zinc layer gradually erodes over decades, especially in polluted or ocean air. If the coating is bruised physically, rust quickly invades through any break, and it needs to be cleaned off and resurfaced with specialized paint, just like ordinary steel. Isn’t there anything better?
Enter stainless steel. For 2,300 years, starting in ancient China, the alchemists of steel alloys have sought a version that would never rust. European metallurgists finally began to succeed in the 19th century. These days, of the 3,500 steel alloys manufactured for various purposes, some 180 are called “stainless.” The term is “stainless” instead of “rustless” because the first widely known usage was for knives that would not stain from the acids in fruit and vegetables. In 1915 the New York Times broke the news that a firm in Sheffield, England,
has 'introduced a stainless steel, which is claimed to be non-rusting, unstainable, and untarnishable…. The initial cost of articles made from this new discovery, it is estimated, will be about double the present cost, but it is considered that the saving of labor to the customer will more than cover the total cost of the cutlery in the first twelve months.13The magic ingredient this time is the metal chromium. When it makes up more than eleven percent of the alloy, the chromium forms a micro-thin layer of chromium oxide that is stable and highly corrosion-resistant. The protective coating has the ultimate in self-healing properties because it forms instantly whenever the underlying steel is exposed to the air. Thus stainless steel requires even less maintenance than galvanized steel.
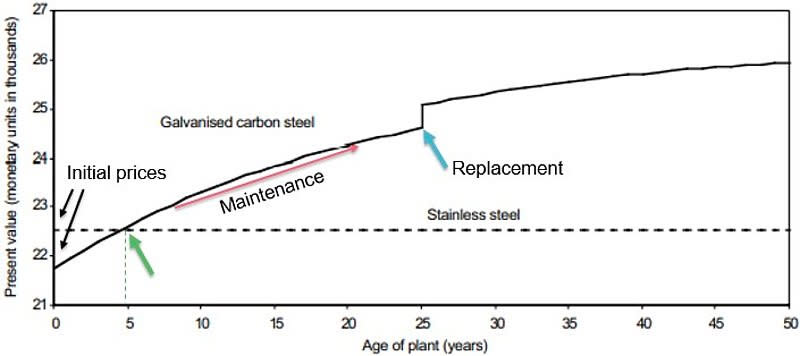
Galvanized steel is cheaper to buy than stainless steel, but as soon as a few years go by, the maintenance costs for galvanized add up, making stainless the less expensive option over time. This diagram compares the “Life Cycle Costing” of two manhole covers for water storage over 50 years.

This galvanized steel pipe rusted so badly in only seven years that it blocked the water supply at a site in Texas. It was attached to a brass valve that caused the pipe to lose its zinc protective coat through galvanic corrosion, and the exposed steel rusted rapidly. If the stainless steel pipe shown above it had been used instead, the problem would not have occurred. The short piece of galvanized pipe cost $3.26. The identical piece of stainless pipe would have cost $3.69.
Comparing stainless to galvanized steel, the stainless is more expensive, stronger, and far more resistant to corrosion over time. It is usually the best choice for particularly corrosive environments such as saltwater and for uses such as medicine and food handling that have to be highly sanitary. The shininess of stainless is considered a feature for its looks and because it can be cleaned as perfectly as glass.
However, stainless steel still requires some maintenance, even in ordinary use. Experts advise that dirt and other contaminants that accumulate on the surface can lead to corrosion, so it is vital to keep it clean. But be aware that detergents, alcohol-based cleaners, and steel wool scrubbers can damage the chromium oxide protective coating, so stick with soap and water. And don’t let stainless have direct contact with certain other metals such as aluminum or galvanized steel, because it will cause them to weaken from galvanic corrosion. (Metals corrode by losing electrons to “nobler” metals in the galvanic series. Zinc loses electrons to iron, which loses electrons to copper, and so on.)
Only gold and platinum are corrosion-free. All the other metals require nuanced techniques to protect them. To meet the specialized needs of customers looking to protect their vehicles, boats, buildings, machinery, tools, or weapons, the online Rust Store offers over 3,000 different products dedicated to preventing or removing rust.14
What makes corrosion so pernicious is that the process is slow, incessant, usually out of sight, and it metastasizes like cancer. How that plays out over time can be learned from the saga of what happened to the Statue of Liberty. The 150-foot statue in New York Harbor was a gift from the French in 1886. It was made of 80 tons of copper sheets riveted to 135 tons of wrought iron armature.
Gustav Eiffel, who designed the Eiffel Tower as well as the iron skeleton for the Statue of Liberty, knew that galvanic corrosion would attack the iron anywhere it touched the copper.15 His design kept the metals separate with a layer of asbestos soaked in shellac, but that solution only made the problem worse because, over time, the asbestos layer became a sponge soaking up highly conductive saltwater from the marine environment around the statue, and that accelerated the galvanic corrosion. The rusting iron put so much stress on the rivets that many popped out. Eiffel’s misuse of asbestos was just the first of a century-long procession of maintenance mistakes.
The core problem was administrative. The statue was first placed in the care of the US Lighthouse Board (part of the Treasury Department), then the War Department in 1901, then the National Park Service in 1933. The directorship of the site was a hardship post that few would suffer for long, so management kept changing. When there is no continuity of custody, continuity of scrutiny and maintenance is nearly impossible. Neglect becomes the norm.

Popped and corroded rivets were visible in the toes of Lady Liberty’s right foot. Each open rivet hole let more salt-laden water into the spots where the copper skin was attached to the iron armature, hastening galvanic corrosion of the iron.
Attempts to deal with the popped rivet holes and the destructive moisture they let in led to a series of stop-gap measures. In 1911, a coating of coal tar was applied everywhere on the interior to fill in the leaks. Some of it leaked through the rivet holes and discolored the outside of the statue. Over the years, eight additional layers of various kinds of paint trapped moisture between the copper and iron and concealed the escalating corrosion.
Never paint rust.
The extent of the damage only became apparent in 1981 when the findings of a comprehensive investigation were reported. According to Waldman’s Rust book, the engineers found that “one-third of the statue’s twelve thousand rivets were loose, damaged, or missing, and more or less half of the iron frame had corroded.”16 The painted-over problems were so extensive that parts of the statue, such as the upraised arm, were in danger of collapsing. The statue that was regarded as a symbol of America was now a symbol of decay. And its centennial celebration, in 1986, was just five years away.
Restoring the statue became a matter of national honor. Committees of public-spirited citizens raised $277 million (about $1.5 billion these days.) Committees of preservation engineers devised schemes to repair the corrosion damage at a scale never before attempted, and they laid out strategies to keep the statue corrosion-free in the future. Since the goal was historic preservation, the original copper skin of the statue had to be treated as inviolate. Scaffolding on the exterior wasn’t allowed even to touch the skin at any point.
So they built the world’s tallest free-standing scaffolding—305 feet from the pedestal’s base to the tip of the torch. When removing the eight layers of paint on the inside of the copper skin proved to be impossible with traditional techniques, the engineers invented a cryogenic solution. Thirty-five hundred gallons of liquid nitrogen were sprayed on the paint to make it so cold it lost its grip and came off in sheets. Getting the coal tar off, however, required something even more drastic. The eventual solution was a combination sandblaster and vacuum cleaner to scour all the copper with 40 tons of baking soda, followed by a complete rinse with vinegar.
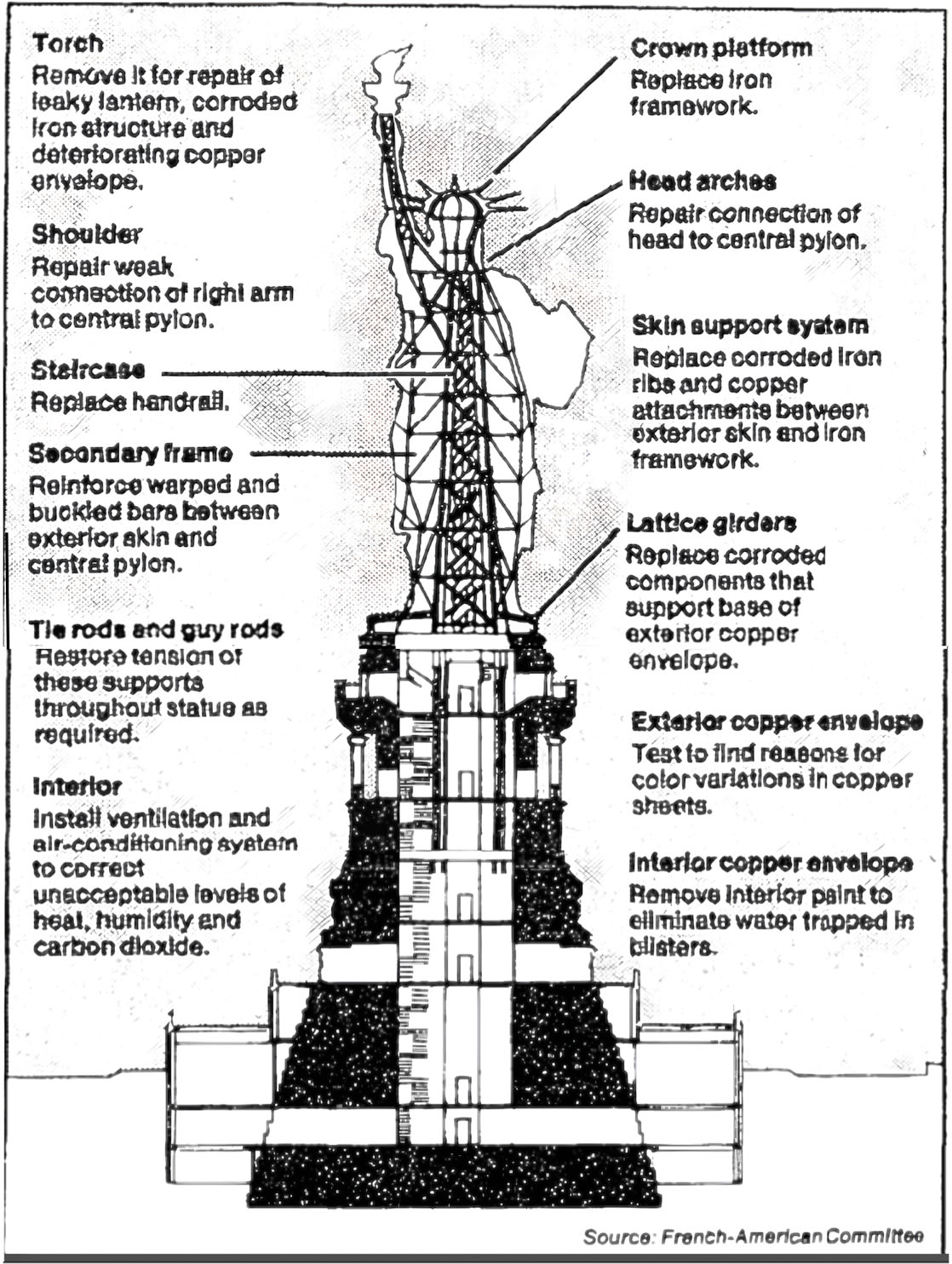
Corrosion problems dominated nine of the twelve major projects planned for restoring the Statue of Liberty in time for its centennial in 1986. The work had to repair all the past corrosion damage in the statue and do so in a way that would prevent corrosion in the future.

Looking down the folds of the statue’s toga, the uniqueness of each of the 1,825 iron armature bars bracing the copper skin is readily apparent. Replacing every single bar with a perfect stainless steel replica was a gargantuan task.
As for the colossal iron frame, it was too far gone. Every one of the 1,825 six-foot armature bars uniquely shaped to the curves of the sculpture’s skin had to be replaced with an identically shaped bar of stainless steel. The process of heating, twisting, and pounding each replacement stainless-steel bar into its correct shape made the metal so brittle that it had to be annealed to make it as supple as the wrought iron had been. (The statue’s copper skin moves in the wind, and the armature metal has to move with it.) Annealing required heating each bar to 2,000 degrees Fahrenheit, then quenching it in water and sandblasting it. But annealing destroys the chromium oxide layer that makes stainless steel rust-proof. To get the layer back, each annealed bar had to be scrubbed with detergent, rinsed, bathed in nitric acid for 30 minutes, rinsed again, boiled in deionized water, air dried, and tested in a copper sulfate solution before it could be wrestled to its unique place high up in the sculpture, taped with Teflon for protection against galvanic corrosion, and refastened to the skin. Molecule by molecule, the statue’s giant metal skeleton was refreshed.
The process was so labor-intensive it took six months of 24-hour workdays to complete.17 (The ongoing maintenance budget for the statue these days, I’m told, is about $6 million a year: $5 million for managing public access, $1 million for physical maintenance. And any year the $1 million is not entirely spent, the remainder is banked so that the fund will become sufficient over time to pay for a major overhaul.)
Having a hard, extremely public deadline forced the project to finish on schedule. On July 4, 1986, the centennial celebration was attended by millions of people, 40,000 boats, and two Presidents—Ronald Reagan of the US and François Mitterrand of France—while a third of the world’s population watched on TV. The night was lit up with twenty tons of fireworks—the largest display in history at that time. They provided a thundering light show for the US Marine Band playing “The Stars and Stripes Forever” and a chorus roaring “Glory, glory, hallelujah.”
It was just a maintenance project.

July 4, 1986, New York Harbor. The Statue of Liberty is rebuilt from within with stainless steel. Because of a blunder in the original construction followed by a century of botched maintenance, the repair cost $1.5 billion.
(End of CORROSION digression)
___________________________